Understand MIPS for your Practice
Maximize the use of your EHR
Maximize the use of your EHR
Meaningful Use helped hundreds of thousands of independent physicians afford adding EHR to their practices. Since 2015, much has changed. The primary goal with MACRA is to improve care for Medicare patients. MACRA shifts the payment system from volume to value and results.
As an ONC 2015 Cures Update Certified EHR, AdvancedMD software meets and satisfies all the MACRA-related requirements. MACRA (Medicare Access and CHIP Reauthorization Act of 2015) introduces a new value-based reimbursement system called the Quality Payment Program (QPP) with three important changes impacting your Medicare payments.
MIPS/APMs
Created a new framework for rewarding physicians for providing higher quality care. The two tracks of the QPP are:
SGR
Repealed the Medicare sustainable growth rate (SGR) formula that calculated payment cuts for physicians
Consolidates
Parts of three previous individual reporting programs are combined into a single system:
The patient chart displays & confirms data such as patient medications, allergies, problem list, smoking status & test results.
Quality Measures are calculated using ICD-10, CPT, LOINC & SNOMED codes.
24/7 access for your patients to see their health records
Telemedicine can maximize your IA performance category score.
Lets you generate reports of patients based on gender, age, problem/diagnosis & test results.
Once entered into the electronic chart, patient data is retained helping you meet the MACRA objectives.
Exchange patient health data using Clinical Direct Messaging and Record Locator Exchange

You’re not alone. Our MIPS professional services team have the expertise to help guide you through the maze of MIPS to give you the tools to achieve a positive MIPS score.
MIPS (Merit-based Incentive Payment System) is a program measuring an eligible clinician’s performance and comparing it with their peers. MIPS is a budget neutral program; the positive Medicare payment adjustments are funded by those receiving negative payment adjustments. The payment adjustments are applied to Medicare Part B payments for covered professional services two years after the performance period.
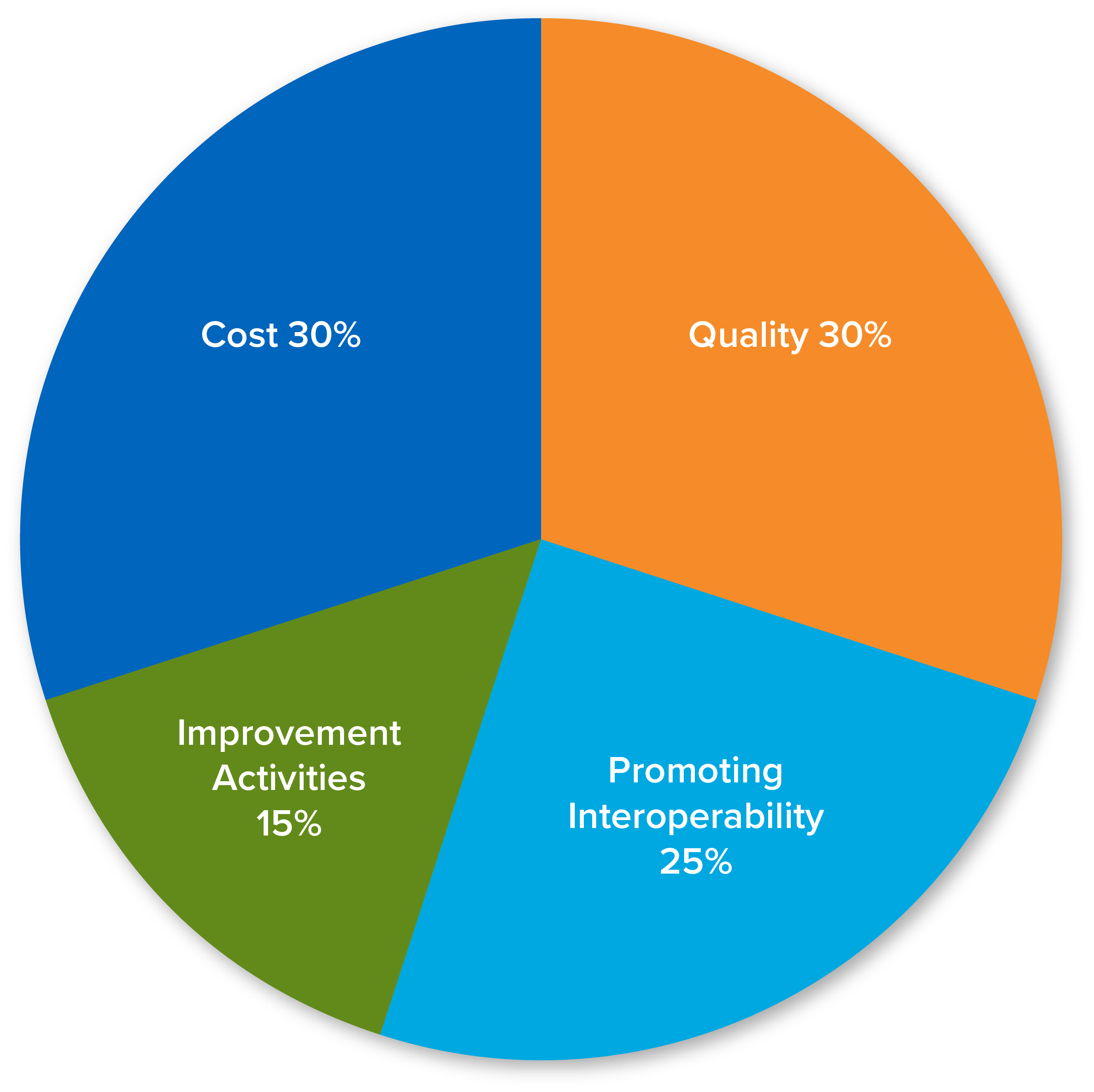
30%
12 months
6 Measures
25%
90 days
15%
90 days
30%
12 months
The second option for participation in MACRA is the APM track where eligible clinicians can apply to and become “Qualified Participants” with an Advanced APM and earn 5% payment incentives. Advanced APMs entities focus on providing high-quality and cost-efficient care—they can focus on specific clinical conditions, care episodes, or patient populations.
CMS’s QPP website has resources to help clinicians decide if participation with an APM is right for them and lists those that are approved for the MACRA QPP. Many previously existing ACOs are on Medicare’s list of Advanced APMs. If you are interested in becoming a QP with an APM we suggest:
